The latest article in our Insight Snapshot series focuses on the NRFC’s Medical Science Priority Area and how it contributes to improving Australia’s health outcomes and national resilience. Click here and here to read the previous article in the series.

Australia has a storied history of medical research, including the development of the electronic pacemaker in the 1920s, ultrasound in the 1960s, multi-channel cochlear implants in the 1970s, and spray-on skin in the 1990s.
Australia continues to perform well in the medical science field relative to its size, with health science research in Australia ranked 7th globally[1] and Australia ranked 9th of 32 high-income countries in the 2024 World Index of Healthcare Innovation[2].
However, ensuring that Australia has a world-leading medical science ecosystem goes beyond just research. It also requires that we better commercialise our innovations to ensure that Australia has robust sovereign medical manufacturing capabilities, secure supply chains, and creates high-skilled, high-value jobs.
In this article, we explain what the NRFC’s Medical Science Priority Area covers, why it matters to the national economy, and explore the investments that the NRFC has already made.
What technologies comprise medical science?
The NRFC’s Medical Science Priority Area spans a wide set of technologies and industries, many of which are already emerging strongly in Australia.
Key technologies in this Priority Area include:
- Biologics and advanced therapies: Next-generation vaccines, mRNA platforms, and cell or gene therapies.
- Medical devices and implants: Advanced prosthetics, imaging equipment, and minimally invasive surgical devices.
- Diagnostics and reagents: Rapid antigen and molecular tests, laboratory consumables, and biosensors.
- Digital and AI-enabled health: Clinical decision support, diagnostic AI, telemedicine platforms, and remote monitoring devices.
- Manufacturing platforms: GMP facilities, bioprocessing equipment, and sterile manufacturing lines.
Each of these has significant potential to lift productivity, reduce import dependence, and build a resilient health economy. The NRFC’s Medical Science Priority Area does not cover cosmetics, veterinary products, or health foods.
Why it matters for Australia
The COVID-19 pandemic highlighted Australia’s reliance on imported vaccines, diagnostics, and critical medical supplies. Building sovereign capability in medical science is therefore not only an industrial opportunity but also a matter of national resilience and security.
The sector also represents one of the fastest-growing global markets, with the medtech (medical devices and technologies for patient care) and medical biotech (using biological processes to develop health-related products) industries expected to be worth trillions of dollars globally by 2030.
For Australia, this means opportunities to:
- translate world-class research into local manufacturing
- grow new, export-oriented businesses in high-value niches
- create thousands of high-skilled jobs across research, production, regulatory affairs, and advanced manufacturing
- reduce supply chain risk and ensure Australians have secure access to essential health technologies.
The growth opportunity
Australia is already recognised for its world-class biomedical research and clinical trial capacity. Yet too often, breakthroughs are commercialised overseas, with Australia re-importing finished products. The NRFC’s investment strategy aims to close this gap and anchor more of the value chain here in Australia.
Global market data underscores this opportunity. The value of the medicines market is forecast to reach more than US$2.3 trillion globally by 2030[3], while the medical technology market is expected to grow at a CAGR of 6.99% between 2025 and 2030 to US$0.96 trillion[4]. Locally, the health and medical research sector contributes over $7 billion to GDP annually[5], and Australia’s strong regulatory environment and public health system make it a trusted environment for scaling trials and new products.
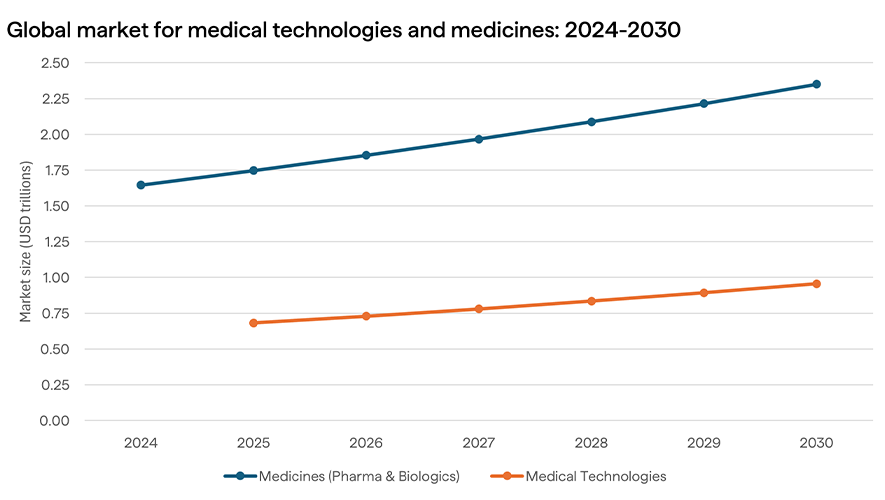
The potential is not only economic but also strategic. Having sovereign control over vaccine platforms, diagnostic reagents, and medical device manufacturing means Australia is better prepared for future health crises and less dependent on fragile global supply chains.
What’s next?
Medical science is a cornerstone of the NRFC’s mission to rebuild Australia’s industrial strength. By anchoring sovereign capability in therapeutics, diagnostics, and devices, the NRFC is helping to create an economy that is both more innovative and more resilient.
In addition to the investments in the Medical Science Priority Area that the NRFC has already made, we are actively assessing investments in Good Manufacturing Practice (GMP) facilities, diagnostics manufacturers, and novel therapeutic developers that can help build sovereign medical capability.
Future articles will take a closer look at emerging areas within the NRFC’s Medical Science Priority Area, such as advanced biologics, AI-enabled devices, and precision diagnostics, and explore the types of projects the NRFC is seeking to support. We will also showcase more case studies of funded projects, outlining the jobs created, the partnerships formed, and the regional impacts delivered.
NRFC investment case studies

Brandon Capital
The NRFC has invested $150 million in the BB6 Fund of Brandon Capital, a leading life sciences venture capital firm that invests in early-stage and high-growth Australian medical science companies.
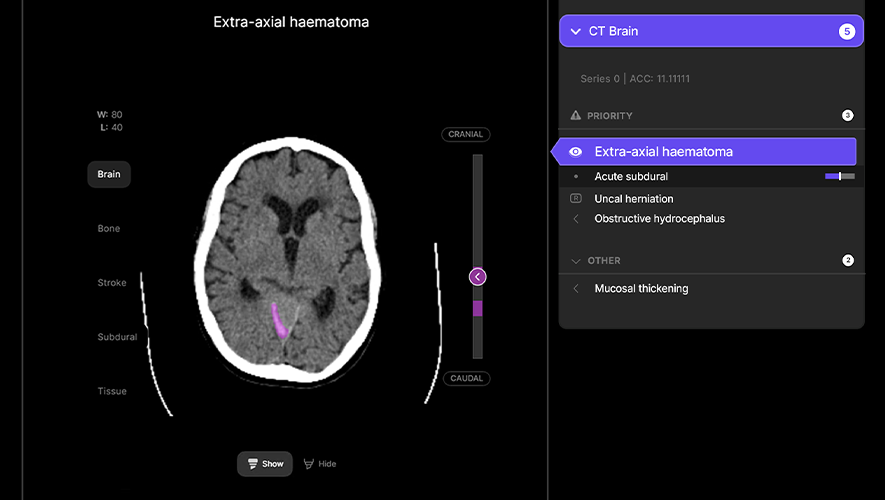
Harrison.ai
The NRFC has invested $32 million in Harrison.ai, a global healthtech company that uses AI to review CT scans and X-rays to support the detection and diagnosis of serious medical conditions.

PolyActiva
The NRFC has invested $27 million in Polyactiva, a biotechnology company pioneering a novel drug delivery technology designed to improve treatment outcomes for patients with ocular conditions.
Get in touch
Do you have an investment-ready proposal in the Medical Science Priority Area? Read our investment guidance and then contact the NRFC to discuss if your project is eligible for funding and how we can work together to build Australia’s sovereign medical science capability.
[1] Department of Industry, Science and Resources, 2024, Medical Science Co-Investment Plan: Building manufacturing capabilities for the future, https://www.industry.gov.au/publications/medical-science-co-investment-plan(Opens in a new tab/window)
[2] The Foundation for Research on Equal Opportunity, 2024, World Index of Healthcare Innovation, https://freopp.org/world-index-of-healthcare-innovation/(Opens in a new tab/window)
[3] Grand View Research, 2024, Pharmaceutical Market Size, Share & Trends Analysis Report by Drug Type (Branded, Generic), by Product (Prescription, OTC), by Region, and Segment Forecasts, 2024–2030, https://www.grandviewresearch.com/industry-analysis/pharmaceutical-market-report(Opens in a new tab/window)
[4] Mordor Intelligence, 2024, Global Medical Device Technologies Market Industry Report, https://www.mordorintelligence.com/industry-reports/global-medical-device-technologies-market-industry(Opens in a new tab/window)
[5] Department of Industry, Science and Resources, 2024, Medical Science Co-Investment Plan: Building manufacturing capabilities for the future, https://www.industry.gov.au/publications/medical-science-co-investment-plan(Opens in a new tab/window)

